Just this past week I completed a new (automated) synthesis of CsNbOB
2O
5; however, the results were a little perplexing. I followed the directions of Becker et al. (1995, attached above) fairly closely. However, it appears that a different phase was stable at high temperature (after soaking at 1000˚C for 20 hours) and that CsNbOB
2O
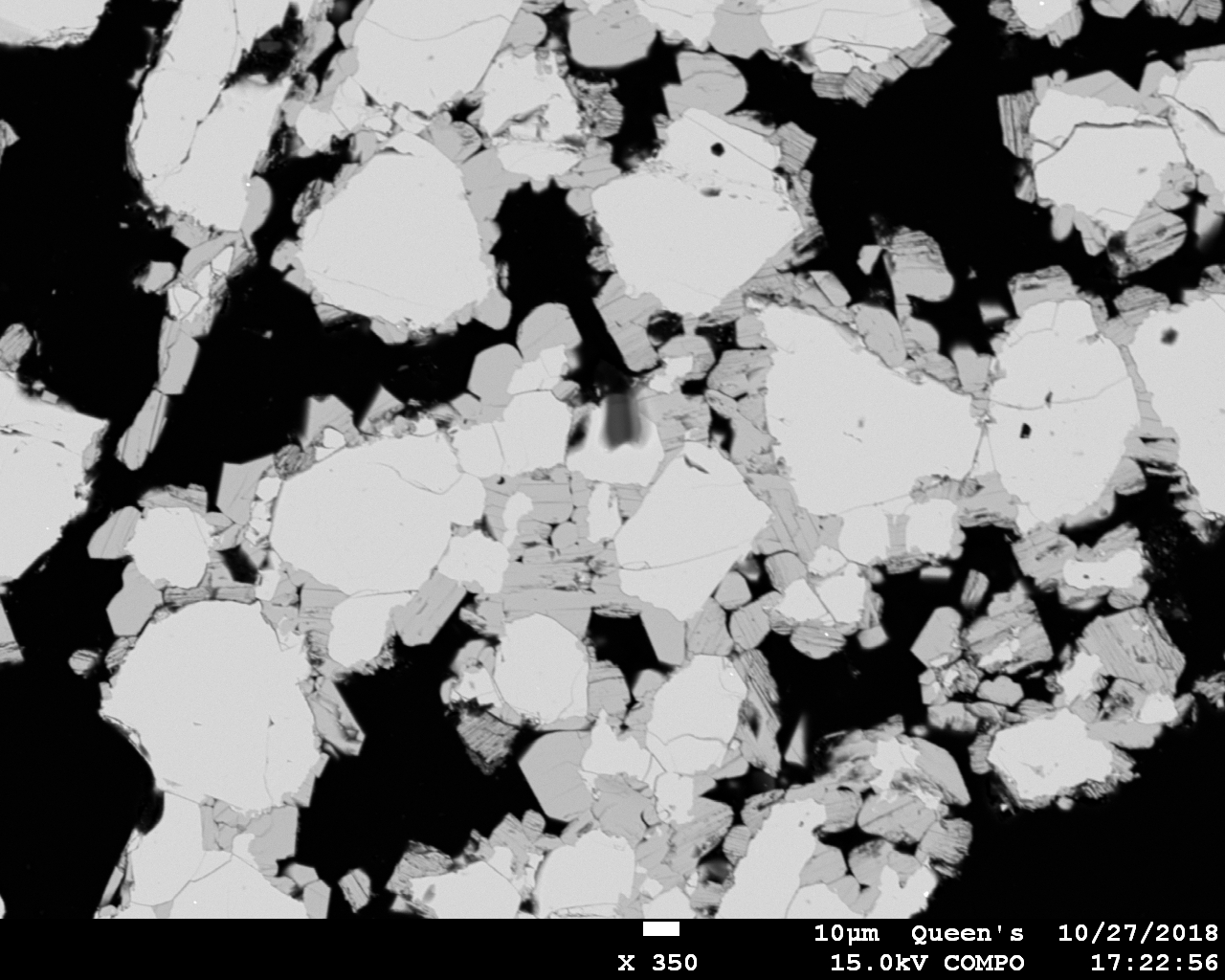
5 only nucleated relatively late at lower temperature while ramping downward at 2.2˚C per hour (more slowly than Becker et al. did). The BSE image below illustrates textural relations between the two phases; the CsNbOB
2O
5 is the finer-grained, darker phase. At first I thought that the high temperature phase might be Cs
2Nb
4O
11, which also has potential as a Cs standard. However, I collected a set of quantitative analyses and found that the formula unit was difficult to establish. The oxide totals were a little low, and so it’s possible if not likely that the phase is also boron-bearing.
I just started a new CsNbOB
2O
5 synthesis run about 30 minutes ago. In this run, ironically, I’ll be trying to emulate the conditions that I used for my first run, in which I adjusted the temperature manually and had to allow the crucible to soak during nights. In my new, automated run, I’ve set the initial soak temperature at 950˚C, followed by a relatively quick ramp downward in temperature and then slower ramps interspersed with 12-hour soaks. I should have results by next weekend.
Lastly, I’ve now tested exposure of CsNbOB
2O
5 to a focused beam at 15 keV and 100 nA for ten minutes. It holds up fantastically even under these conditions:

Dare I try 200 nA?