In our next class, we will attempt again to quant both magnetite and pyrite and again report the results here.
The class quant effort will have to wait until after Thanksgiving, but in the meantime I noticed something interesting about the above scan, shown here again, but with the Fe L absorption edges visible:
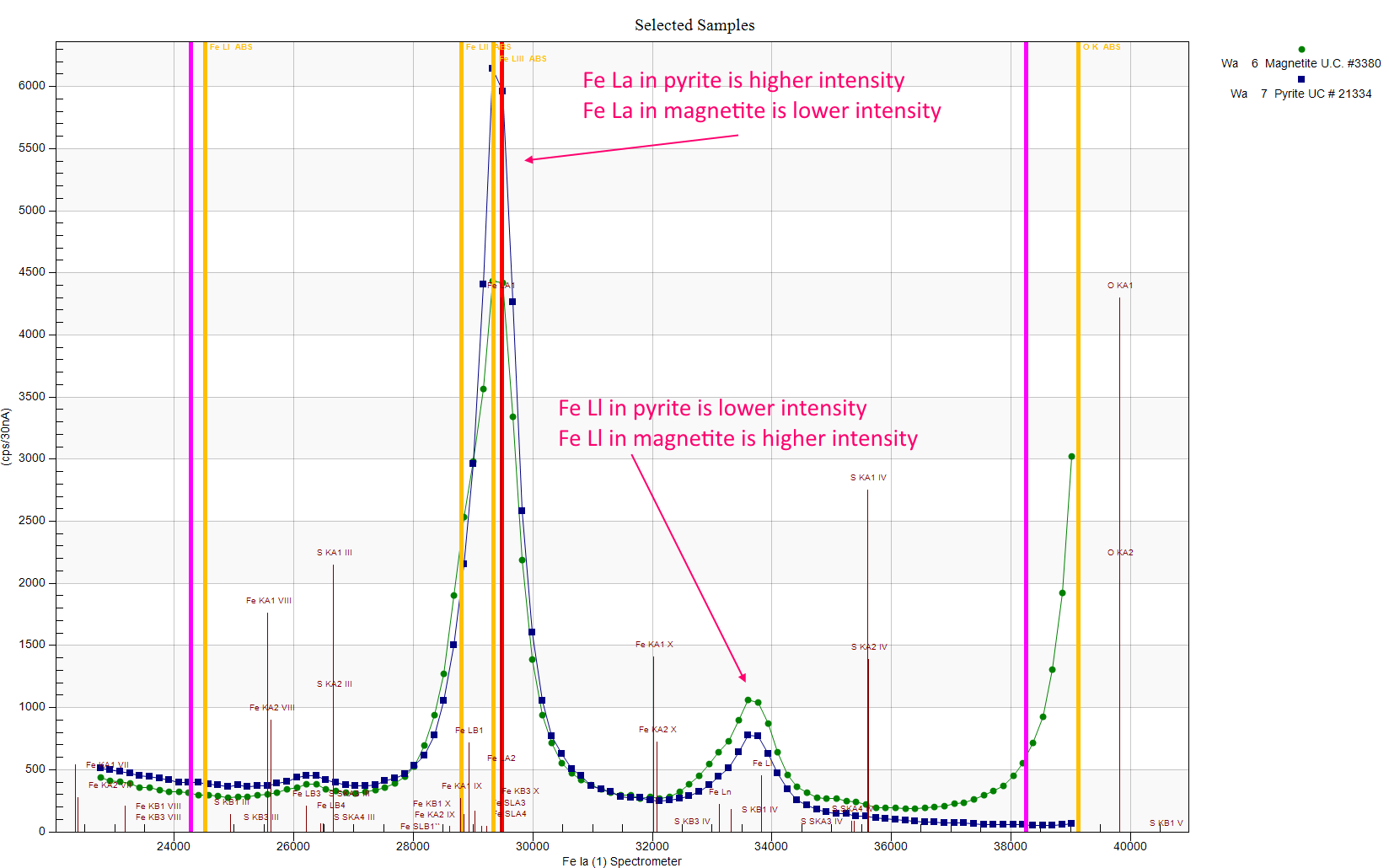
Note that although there is more Fe in magnetite then pyrite, the Fe La line is less intense in magnetite, while the Fe Ll line has the opposite relationship. My understanding is that this is because the Fe La emission energy varies with the chemical state of the Fe (in magnetite vs. pyrite), therefore the Fe L III edge can fall on either side of the Fe La emission line depending on this chemical state. Which in turn affects how much the Fe La emission is absorbed by the Fe L III edge.
That is, if the Fe La emission line is higher energy than the Fe L III edge (Fe L III edge falls to the right of the emission line in wavelength space), the Fe La emission line can excite the Fe L III edge and therefore is readily absorbed. But if the Fe L III absorption edge falls to the left of the Fe La emission line (the Fe emission line is lower energy than the Fe L III edge), the Fe La emission line is not so readily absorbed.
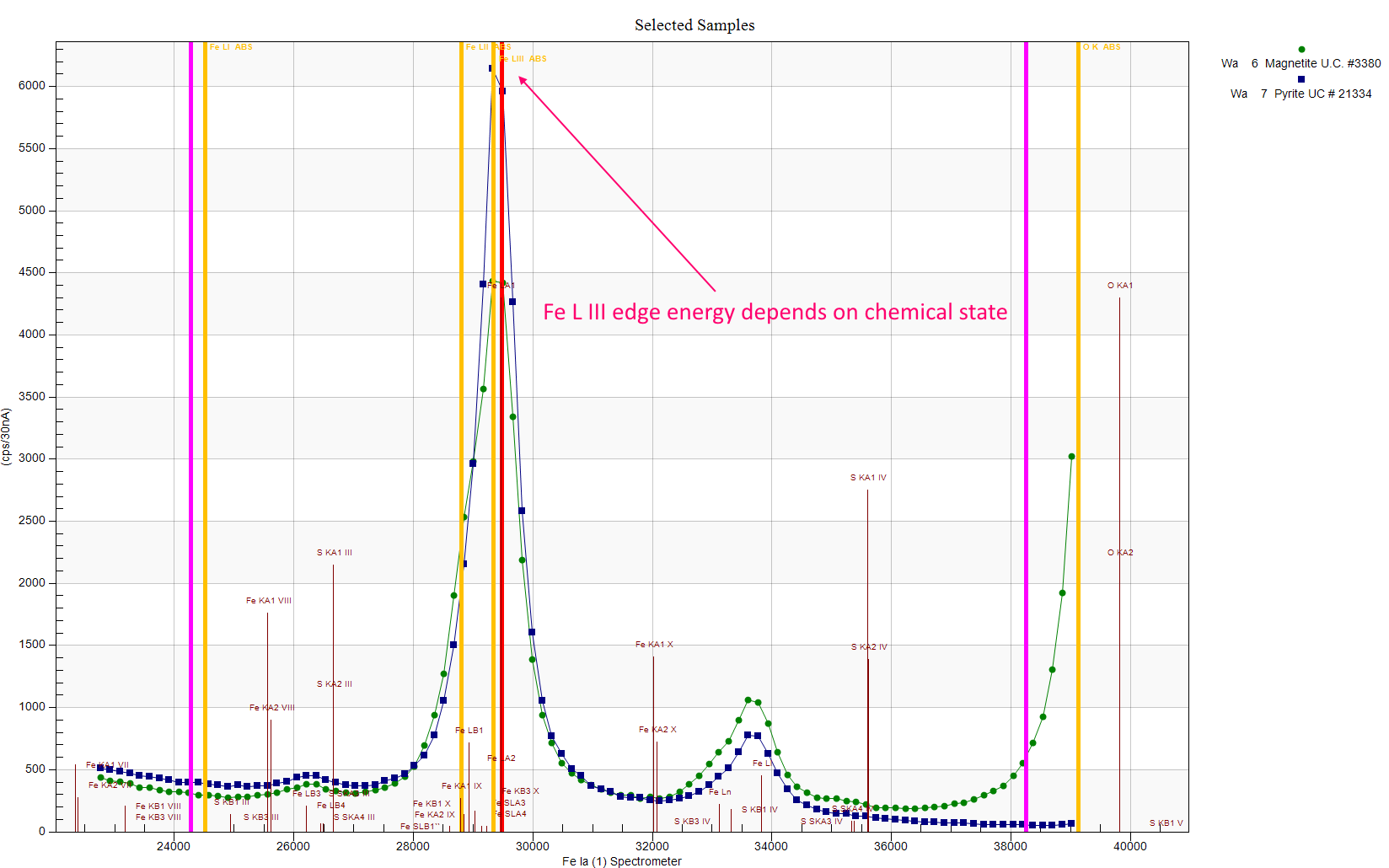
If you look closely one can see that the magnetite Fe La emission line (green symbols) is slightly shifted to the left, which means it's higher energy, which means it would be more absorbed by the Fe L III edge, and that makes sense because the magnetite Fe La intensity is less. I should have the class scan the Fe La region using a TAP crystal and then we would see this energy shift in more detail...
So this is why the Fe Ll emission line is preferred for quantification, rather than the Fe La line.
Interesting stuff.
john