Thank you Mike and Ben for your input. We are quite interested in this as we found that there was enough variation in the carbon coats on a sample vs the standard that the analytical totals were wacky, whereas the stoichiometry was perfect (plagioclase). Inputing different coat thickness values in PfE (from StrataGem k-ratios) saved the day. However, we noted that the thickness in StrataGem (which uses mass thickness) is a variable dependent upon density. So getting an accurate density would be rather useful.
Hi John et al.,
I ran into this same carbon coat density question yesterday on a metallurgical sample I am running for another university.
Basically it's a Ti-Nb couple and quite interesting because, for this system the diffusitivities (is that the correct word?) are different by around 5 orders of magnitude, so Nb diffuses readily into Ti, but Ti will not diffuse into Nb. Really weird.
Anyway, the sample is uncoated, but the standards are coated with our usual 20 nm of carbon. So when I went to do the quant I got this output:
Un 4 Ti-Nb boundary, Results in Elemental Weight Percents
ELEM: Ti Nb O
BGDS: EXP LIN EXP
TIME: 40.00 40.00 40.00
BEAM: 30.01 30.01 30.01
ELEM: Ti Nb O SUM
210 -.009 101.534 1.173 102.698
211 -.008 102.291 1.260 103.544
212 -.001 101.548 1.245 102.792
213 -.023 101.994 1.244 103.215
214 .026 101.660 1.221 102.908
215 .005 102.474 1.201 103.679
216 .063 102.108 1.287 103.458
217 .022 102.335 1.279 103.636
218 -.006 101.594 1.209 102.797
219 .043 101.464 1.317 102.824
220 .003 101.527 1.241 102.771
221 -.013 102.022 1.305 103.314
222 -.001 101.583 1.252 102.833
223 .030 101.848 1.171 103.049
224 .019 101.799 1.305 103.123
225 .035 102.506 1.309 103.849
This is using an 8 keV electron beam because they want the best spatial resolution they can get (we're doing another run tonight at 6 keV to try and further improve the spatial resolution and also to further reduce the SF effects).
Anyway, the above output clearly shows the need to specify the carbon coat for the standards and turn it off for the unknown. For the unknown this is easily done from the Calculation Options dialog by unchecking the "Use Unknown Conductive Coating" checkbox. For the standards one needs to use the Standard | Edit Standard Coating Parameters menu, but by default they are set to 200 nm of carbon at 2.1 gm/cm3.
Then one has to go into the Analytical | Analysis Options dialog and explicitly turn on the conductive coating correction by checking these two checkboxes:
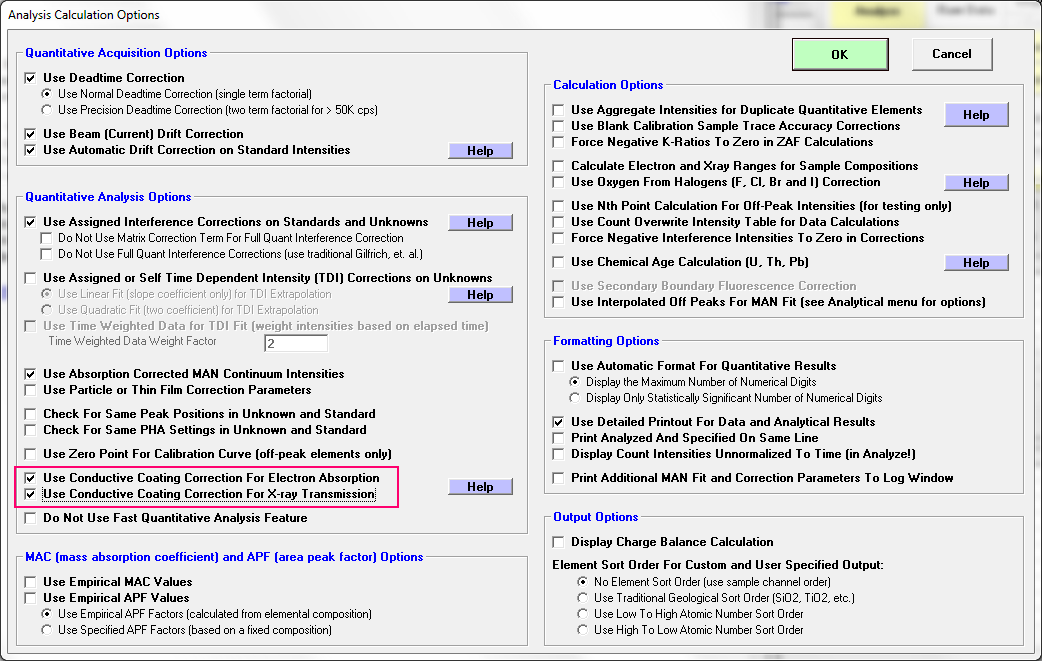
Once that is done we should be OK (or should we?), and we now obtain this analysis:
Un 4 Ti-Nb boundary, Results in Elemental Weight Percents
ELEM: Ti Nb O
BGDS: EXP LIN EXP
TIME: 40.00 40.00 40.00
BEAM: 30.01 30.01 30.01
ELEM: Ti Nb O SUM
210 -.008 95.301 1.024 96.316
211 -.007 96.010 1.099 97.102
212 -.001 95.313 1.086 96.398
213 -.021 95.731 1.085 96.795
214 .024 95.418 1.065 96.508
215 .004 96.183 1.047 97.234
216 .057 95.838 1.122 97.017
217 .020 96.051 1.116 97.187
218 -.005 95.357 1.054 96.406
219 .040 95.232 1.149 96.421
220 .003 95.293 1.083 96.379
221 -.011 95.756 1.139 96.883
222 -.001 95.345 1.092 96.436
223 .028 95.596 1.021 96.645
224 .017 95.547 1.138 96.703
225 .032 96.210 1.142 97.384
So now our analysis is low! Of course these coating effects are significantly amplified when running at low voltage (or low overvoltage), but we're pretty sure that the coating on our standards is close to 20 nm based on the color on polished brass as discussed here:
http://probesoftware.com/smf/index.php?topic=921.msg7063#msg7063So then there's the density question and the consensus above seems to be that the evaporated deposited coatings are often less dense than their "accepted" values. So I changed the carbon density from 2.1 to 1.4 and voila:
Un 4 Ti-Nb boundary, Results in Elemental Weight Percents
ELEM: Ti Nb O
BGDS: EXP LIN EXP
TIME: 40.00 40.00 40.00
BEAM: 30.01 30.01 30.01
ELEM: Ti Nb O SUM
210 -.008 97.376 1.072 98.439
211 -.007 98.101 1.151 99.245
212 -.001 97.389 1.137 98.524
213 -.022 97.816 1.136 98.931
214 .025 97.496 1.116 98.637
215 .004 98.277 1.096 99.378
216 .059 97.925 1.175 99.159
217 .021 98.143 1.168 99.332
218 -.005 97.433 1.104 98.532
219 .041 97.307 1.203 98.550
220 .003 97.368 1.134 98.505
221 -.012 97.842 1.192 99.022
222 -.001 97.422 1.143 98.564
223 .029 97.677 1.069 98.775
224 .018 97.629 1.192 98.838
225 .033 98.306 1.195 99.534
You gotta admit, that's pretty impressive...