Hi John,
Here is an example of an analysis (PAP model, accelerating potential = 15 kV, takeoff angle = 40 deg.) of pure magnetite using a pure magnetite standard. I’ve assumed that all Fe in the unknown is present as FeO. Under “Calculation Options,” I’ve selected “Calculate with Stoichiometric Oxygen.”
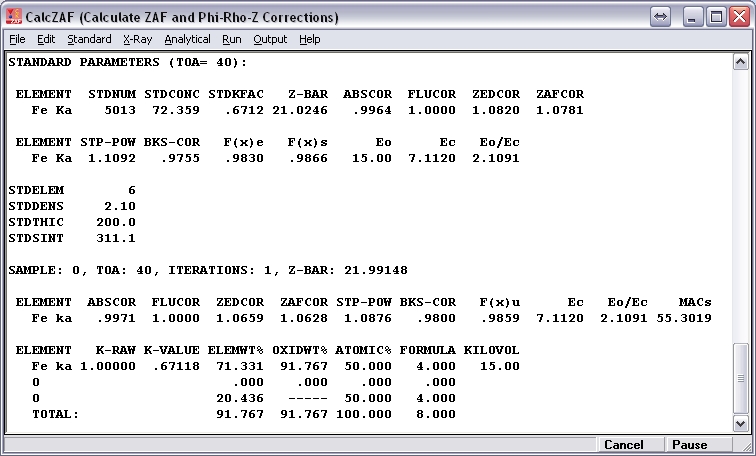
If I then recalculate the result assuming 3 cations and 4 oxygens per formula unit, I obtain 67.996 wt% Fe2O3, 30.591 wt% FeO, and oxide total = 98.586 wt%. (I’ve used molar mass Fe = 55.845 g/mol and molar mass O = 15.9994 g/mol.)
The standard and unknown both contain 72.359 wt% Fe and 27.641 wt% O, and Fe k-raw is precisely one. If it is assumed that Fe is present in the unknown as FeO, then the calculated weight per-cent of oxygen is 20.731, producing an oxide total of 93.090 wt%. However, during the matrix correction iterations, compositions are normalized, and, during the first iteration, the Fe and O contents of the unknown become, respectively, 77.730 and 22.270 wt%. If matrix corrections are determined based on this composition, then, relative to the standard, the Fe atomic number correction (PAP model) is 0.9851 (=1.0659/1.0820) when in truth it should be precisely one. This mostly accounts for the anomalously low wt% Fe in the result since the absorption correction is close to unity.
Why does CalcZAF not include a provision to account for mixed oxidation state of a chosen element during the matrix correction iterations? Or is this somehow possible in CalcZAF, and I just don’t see it?