Finally here are the results for insulating materials measured over a range of beam sizes. I choose synthetic Al2O3, synthetic "ruby" (Al2O3 plus 0.4% Cr) and synthetic MgO. The idea being that pure Al2O3 and Al2O3 doped with 4000 PPM of Cr will have very different electrical conductivity (does anyone know the actual electrical conductivities?). These effects could possibly produce a difference in x-ray intensity due to differences in sub surface charge distribution. Let's test that idea...
Using a Cameca SX50, the O Ka was measured on a PC1 crystal, the Al Ka and Mg Ka on a TAP crystal and the keV was 15 and beam current 30 nA. All materials were carbon coated with 20 nm of carbon and each measurement was performed on a clean area.
The results for synthetic Al2O3 are here:
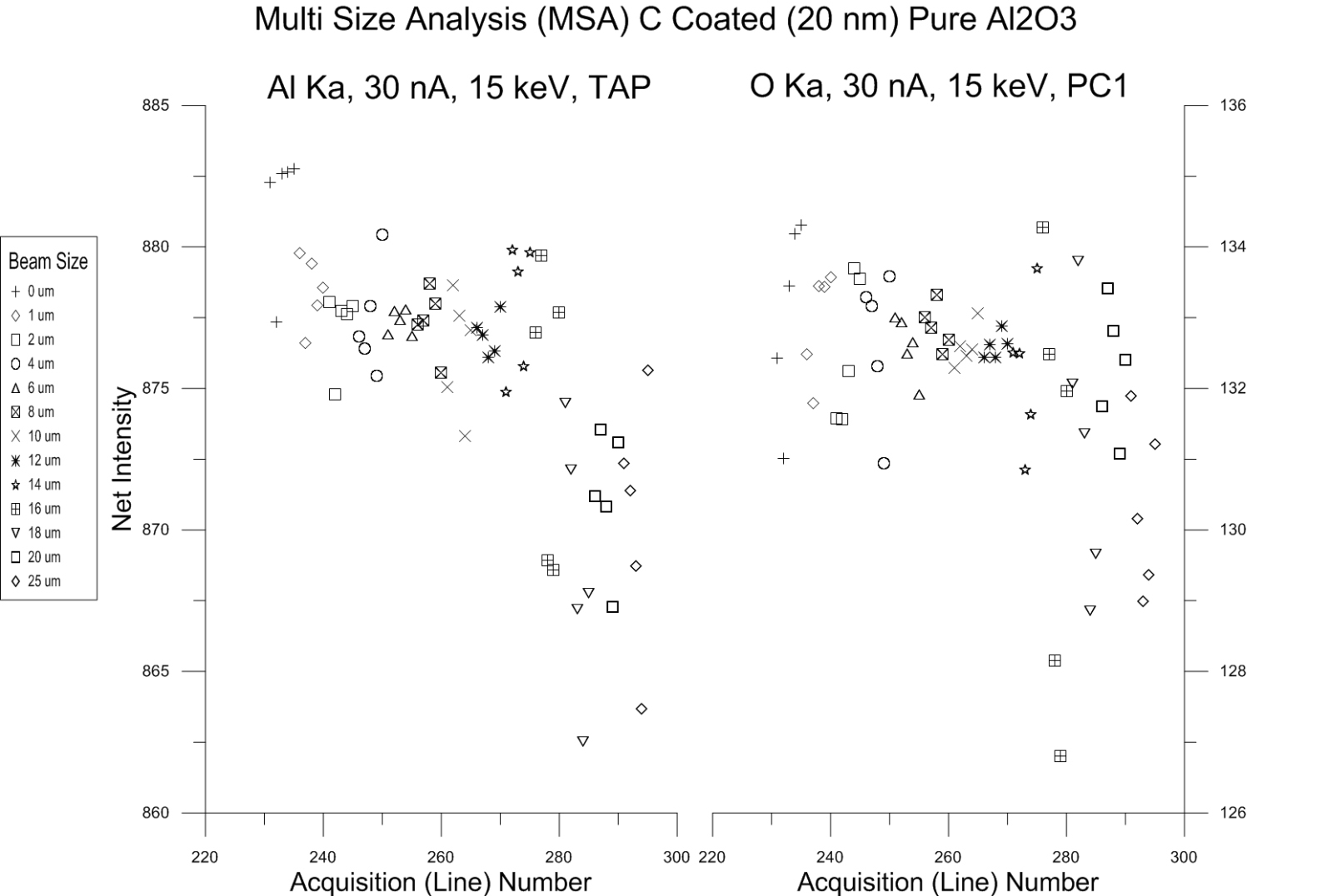
As you can see except for a small intensity drop for Al Ka and O ka at the largest beam sizes of 20 and 25 um, the intensities seem stable over the range of beam sizes. This is not unexpected due to Bragg defocusing effects.
So are we done? Well I thought so until I saw the next plot of synthetic "ruby" (Al2O3 with 0.4% Cr):
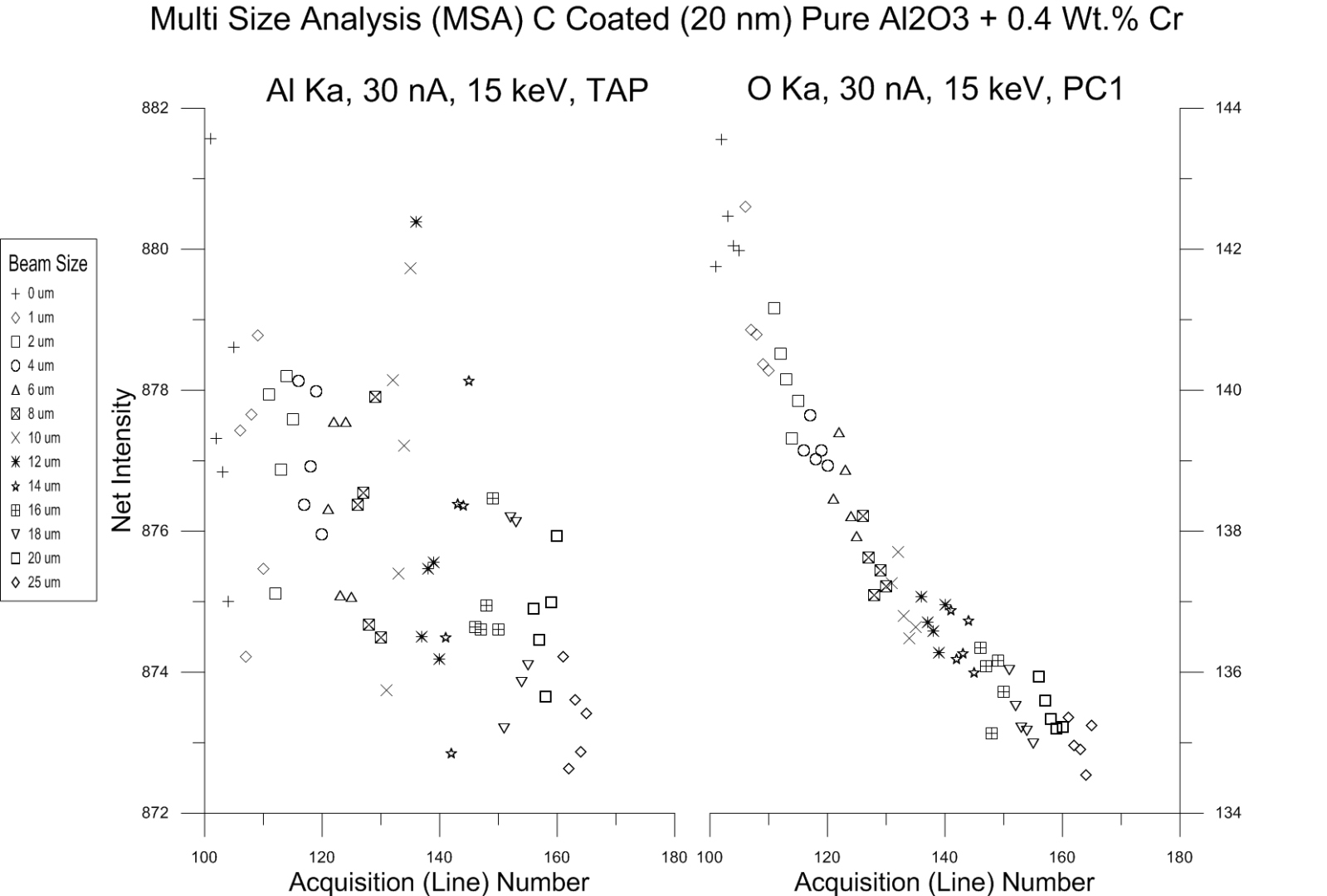
What the heck is going on with the O Ka intensities in "ruby"?
A couple of things to note: first the Al Ka intensity in "ruby" seems consistent, but the O Ka intensity starts at the smallest beam sizes with a significantly higher intensity than pure Al2O3, but by the time the beam is defocused to 20 or 25 um, the intensity is back to that we obtained from pure Al2O3.
I also note that the Cr Ln lines is close to the oxygen line, but why would that make the intensity higher at smaller beam sizes? I am running some wavescans at different beam energies now to look at the peak shapes.
Oh, and the MgO plot? That is attached below. It shows a similar effect for oxygen as the Al2O3 with Cr (but not for Mg Ka!), and yes, the Mg Ka II order in close to O Ka but not that close... so what is going on?