when I was recently at Lehigh, some attendees approached me later being confused about why they should use a program called "CalcZAF". They had just learned that ZAF is the old fashioned way and that phi-rho-z is the way to go. While this misunderstanding was easy enough to clear up, I wondered how much a name like this could stop people from using it or if anyone else shared this first impression.
That is sad, but interesting too.
I guess I should have branded CalcZAF as CalcPRZ from the beginning(!), because the default matrix correction has always been the Armstrong pr(z) model! And of course CalcZAF has always included the XPP and PAP pr(z) methods as well.
As an illustration of the pr(z) methods in CalcZAF, the other day I had some students from OSU down that wanted to measure the Ti to O ratios in some plasma deposited TiO thin films, but they deposited them on an SiO2 substrate. Hmmm, I thought, this could be interesting. Quant oxygen in thin films, on an oxide substrate... but fortunately in a thin film the absorption correction for oxygen K should be pretty small, even in the presence of Ti, which of course highly absorbs O Ka emissions.
First I thought: well if we can keep the interaction volume within the deposited film we can keep the oxygen signal from the SiO2 from our measurements, but unfortunately the film thicknesses were in the range of 30 to 60 nm and we don't have a FEG EPMA, so the lowest the W filament can easily go is maybe 5 keV which precludes the Ti Ka line since the Ti K edge is 4.97 keV, so the overvoltage would be essentially 1.0. We could have used the Ti La emission but given the problems with first row transition L emission peak shapes and absorption edges I figured that might be a bit of a research project all by itself (has any one tried the Ti La emission line in Ti oxides? The Ti Ln emission is missing and I'm assuming that the Ti Ll emission is pretty weak).
So we used the phi-rhi-z curve plot feature in CalcZAF(!) to try and see where the emitted x-rays are coming from. For reference here is what TiO2 looks like at typical EPMA beam energies (15 keV):
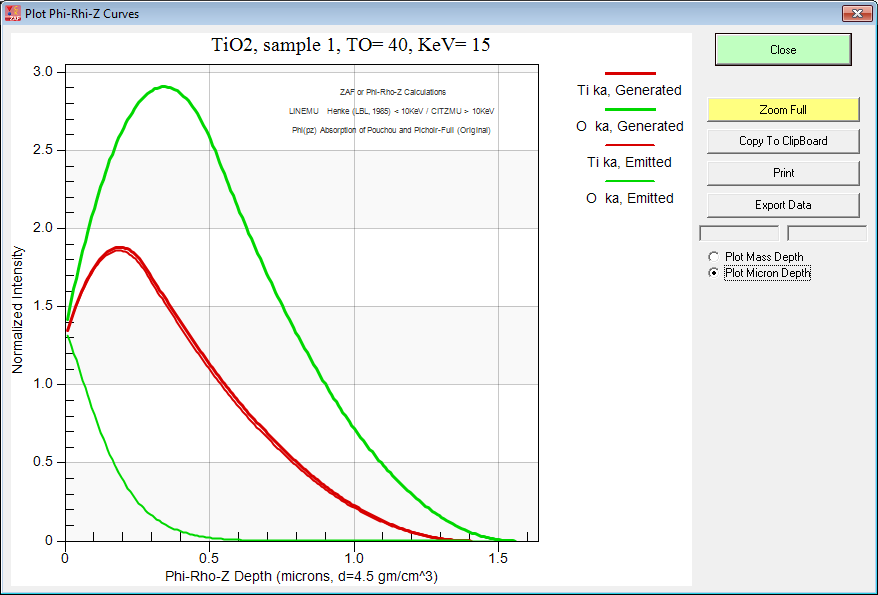
And here is TiO2 at 6 keV:
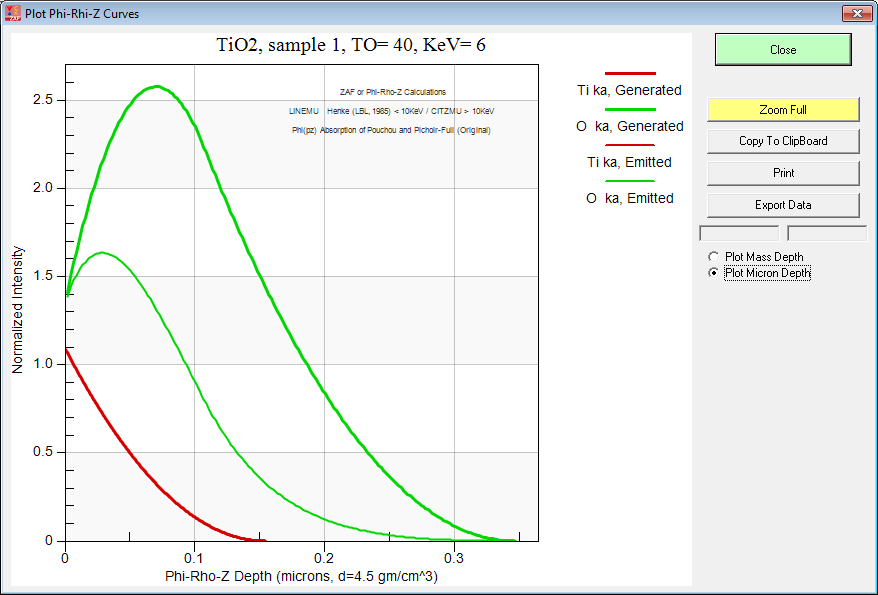
By the way, if you want to do this sort of modeling to decide on your analytical strategy, be sure to utilize either the PAP or XPP methods because they are physically realistic in their depth distribution. After all they were "tuned" using multiple thin film depositions! Just to demonstrate the point here is the Armstrong pr(z) curves for TiO2 at 6 keV:
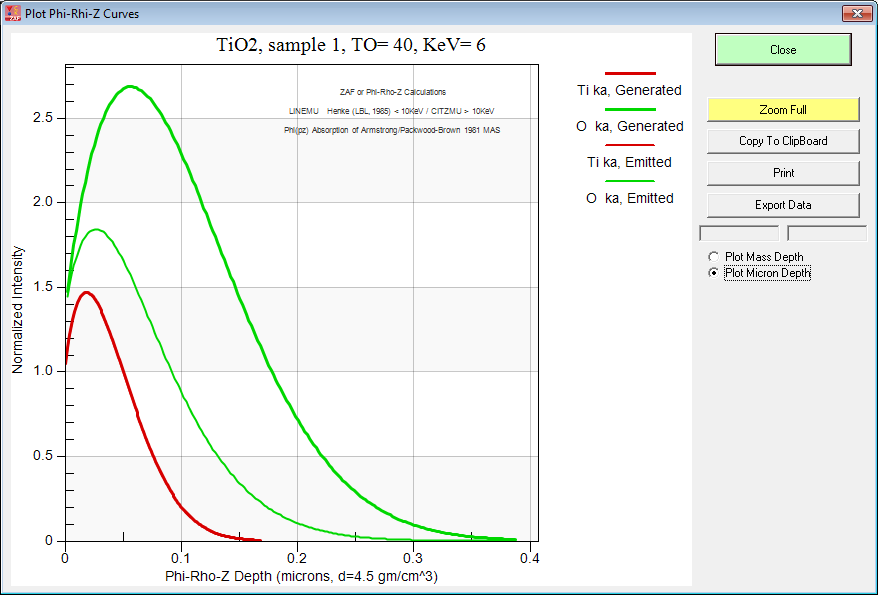
Just a wee bit of difference!
Anyway, even at 6 keV we'll be getting significant oxygen emission from the substrate, and also because the film thicknesses varied over the surface of the deposited films, we decided to treat it as a normal multi-voltage thin film analysis, where we don't know the compositions nor the film thicknesses. Which is what we typically do for thin films, and so we measured the Ti K and O K lines at 6 keV and 8 keV.
I will just say that we got results that were consistent with their models, but I've asked them to re-deposit the Ti-O films on a substrate that does not contain oxygen such as Si or C. They'll probably use carbon substrates because the Si crystal structure will apparently affect their film properties.
In a couple of weeks I'll report a comparison between the two sets of measurements.