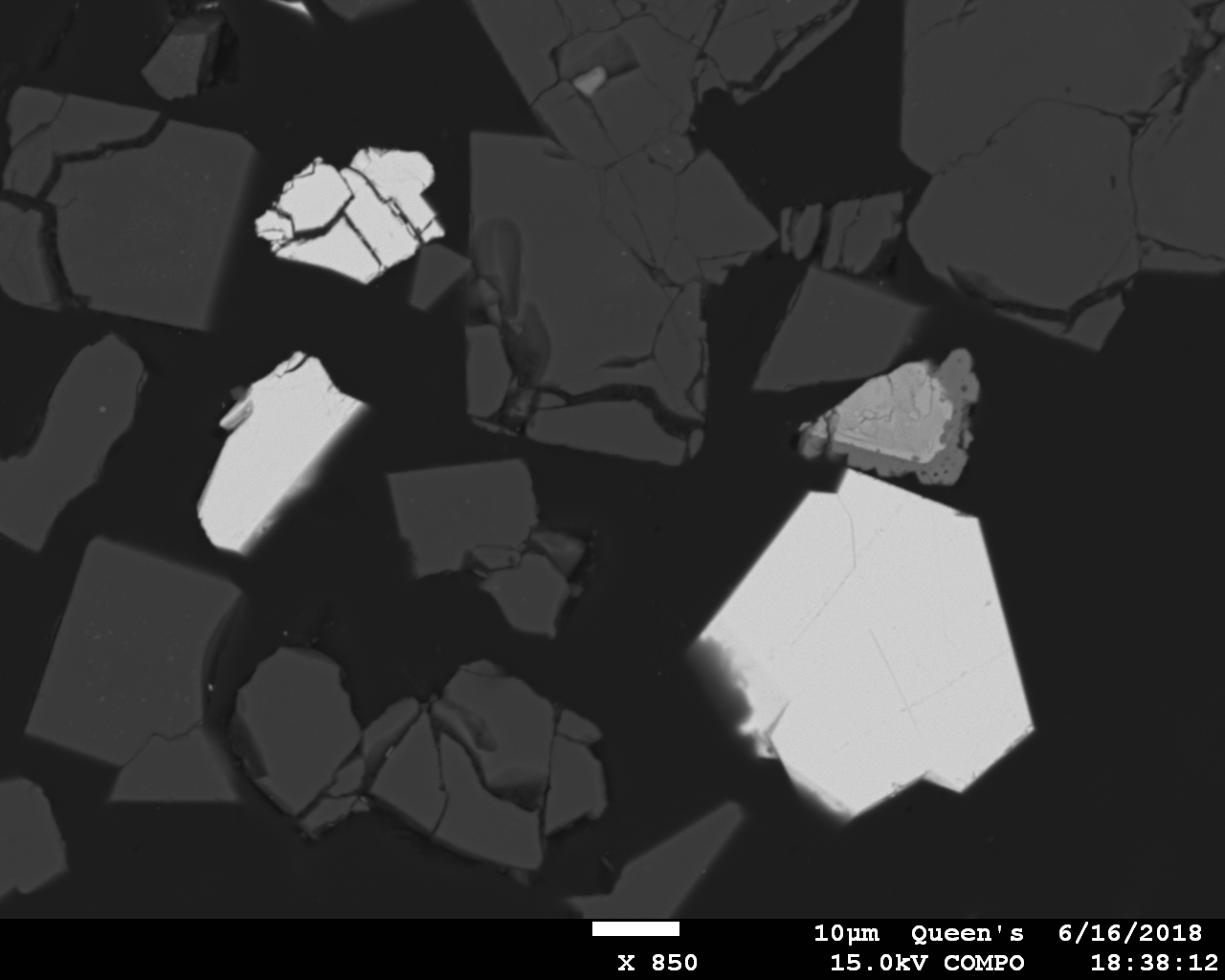
Two weekends ago, in a failed attempt to synthesize CsZr
2(PO
4)
3, I accidentally produced a Cs compound that shows at least some potential. I don’t have access to Pt crucibles, so in lieu of this, I lined a 15 mL alumina crucible with Pt foil and then placed it along with the reactants in a furnace at 700°C for 70 hours. What I didn’t realize was that the platinum would react extensively with the liquid mixture of Cs phosphate and phosphoric acid to produce a distinctively orange Pt-bearing liquid and three crystalline Pt phosphates, two of which are Cs-bearing (the third being PtP
2O
7). In the BSE image below, the (relatively) large, bright grain to the right is the more Cs-rich of the two. Based on a set of 23 analyses, I believe it to be Cs
4Pt(III)
6P
8O
31, which contains 23.0 wt% Cs
2O. It grew on the Pt foil as clusters of deep orange-red crystals that have adamantine luster and appear to have hexagonal(?) symmetry though are nearly equant -- they’re actually quite pretty. Another fractured grain of it is present in the upper left of the BSE image. Just below that grain is a crystal of the other Cs-Pt phosphate, which, based on a set of six analyses, appears to be Cs
2Pt(II)
6P
8O
27. This one, which contains 13.3 wt% Cs
2O, is less common, has a deep olive-brown color in transmitted light (it’s almost opaque), and has near-metallic luster; when carbon-coated it acquires a distinctive blue tint. The abundant dark grains in the BSE image are Al(PO
3)
3, which formed due to reaction of the alumina crucible with the liquid after the Pt foil tore due to excessive thinning.
The Cs
4Pt(III)
6P
8O
31 appears to be relatively stable under the beam. When collecting a set of wavelength scans at 15 kV and 50 nA and with the beam defocused to 10 microns, the absorbed current remained constant for about eight minutes before starting to drop. When I get a chance, I’ll test it in the same manner as I did the pollucite and CsCoPO
4.
I should note that I haven't found any literature at all on Cs-Pt phosphates. The formulas that I wrote above require more than one type of phosphate anion to be present. For instance, the more Cs-rich compound would need to contain both P
2O
7 and PO
4 groups. Alternatively, the formula of each compound could be simplified if the Pt is present in more than one oxidation state.