I’ve come across a compound, Cs
2V
4O
11, that could serve as a useful Cs standard simply because it’s incredibly easy to produce, is not water soluble, is not hygroscopic, and is only moderately beam-sensitive. The attached paper provides some details on the structure of Cs
2V
4O
11. The compound contains 43.65 wt% Cs
2O and 56.35 wt% V
2O
5. When using Cs
2CO
3 and V
2O
5 as starting materials, 47.25 wt% Cs
2CO
3 (dried) and 52.75 wt% V
2O
5 give the proper stoichiometry. Or one can use 48.07 wt% CsCl (dried) and 51.93 wt% V
2O
5. If an excess of Cs
2CO
3 or CsCl is added, then CsVO
3 is produced in addition to the target compound. CsVO
3 is water soluble (though dissolves slowly), and so its presence provides a useful means of loosening the Cs
2V
4O
11 crystals, though some chipping may be necessary. In my run, I used 1.56 g powdered Cs
2CO
3 and 1.48 g powdered V
2O
5, mixed thoroughly with a toothpick. Although I used an alumina crucible to grow the compound, it would probably be fine to use a porcelain crucible. The synthesis is outrageously simple: Just place the mixture in a furnace overnight at ~550°C and then allow it to cool to room temperature within the furnace. That’s it. (I cooled the furnace at 5°C per hour for 20 hours, but I don't know that this is necessary.) In this approach, when using Cs
2CO
3, the volume of the crucible needs to be at least an order of magnitude greater than the volume of the reactants, as the reaction releases CO
2. The resulting intergrown reaction products are 100% crystalline, and so the yield is high. The Cs
2V
4O
11 is orange-brown, while the CsVO
3 is colorless. The crystals range up to several mm in length and possess voids previously occupied by CsVO
3 (with very minor remaining CsVO
3 producing bright spots in BSE):

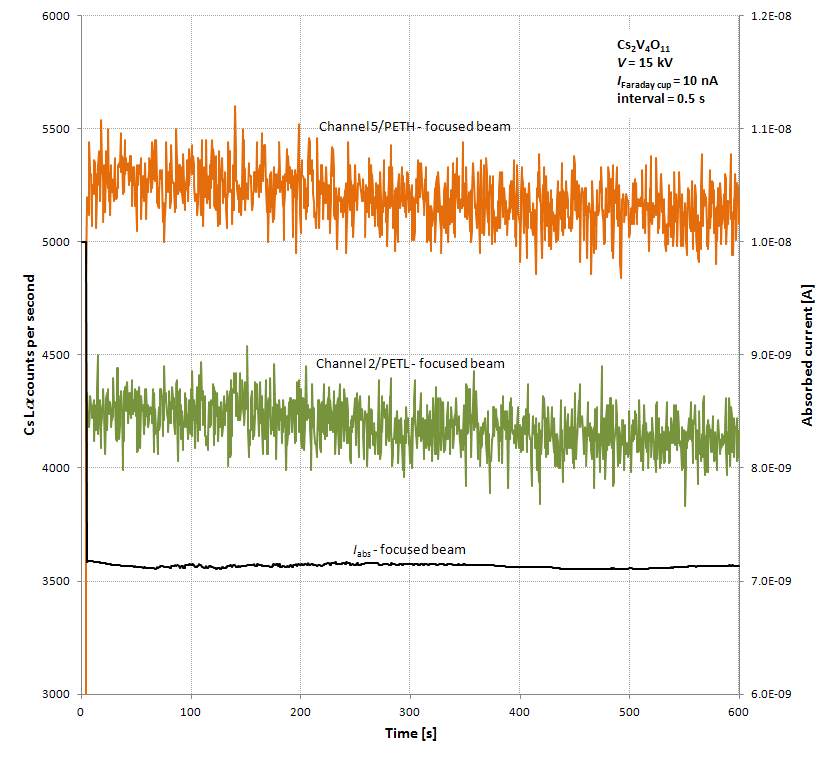
I’ve subjected the material to my usual test of exposure to a focused 15 keV/10 nA beam for ten minutes. The results, shown below, show that the Cs Lα count rate remains essentially constant for perhaps 150 seconds before starting to drop off.
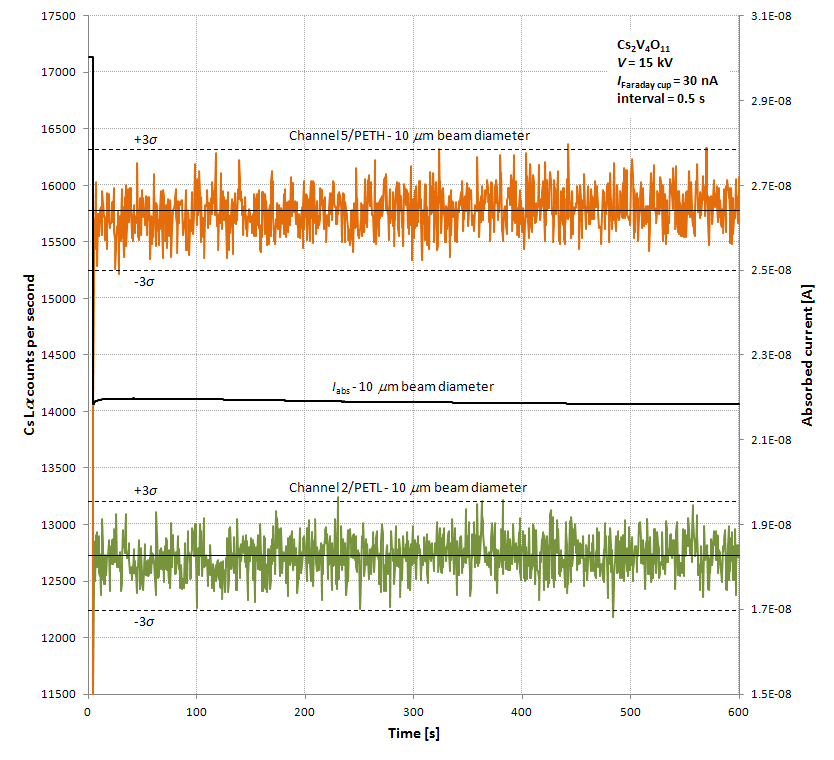
With a 30 nA beam current and the beam defocused to 10 microns, the Cs Lα count rate remains steady within counting error. Note that the apparent slight upward drift in count rate on channel 5 is not present on channel 2. Regardless, just slight defocusing of the beam (to perhaps 5 microns) at 10 nA should prevent beam damage and yield count rates in the few thousands per second on PET.
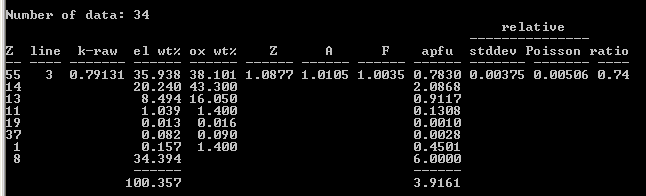
Analyzing my pollucite for Cs while assuming a fixed matrix composition produces the results shown below. Note that Cs L
3,abs is located at ~79.16 mm on PET and at ~172.00 on LiF, so V Kα cannot ionize Cs L
3, but V Kβ can; therefore a small but non-negligible fluorescence correction to Cs Lα must be considered. This is not the ideal situation, but, then, the correction is less than 0.5%. Once again, the atomic number correction dominates the matrix corrections.
PAP/MAC30:
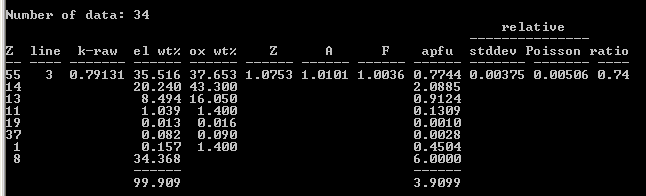
Armstrong/FFAST:
WD spectra are shown here:
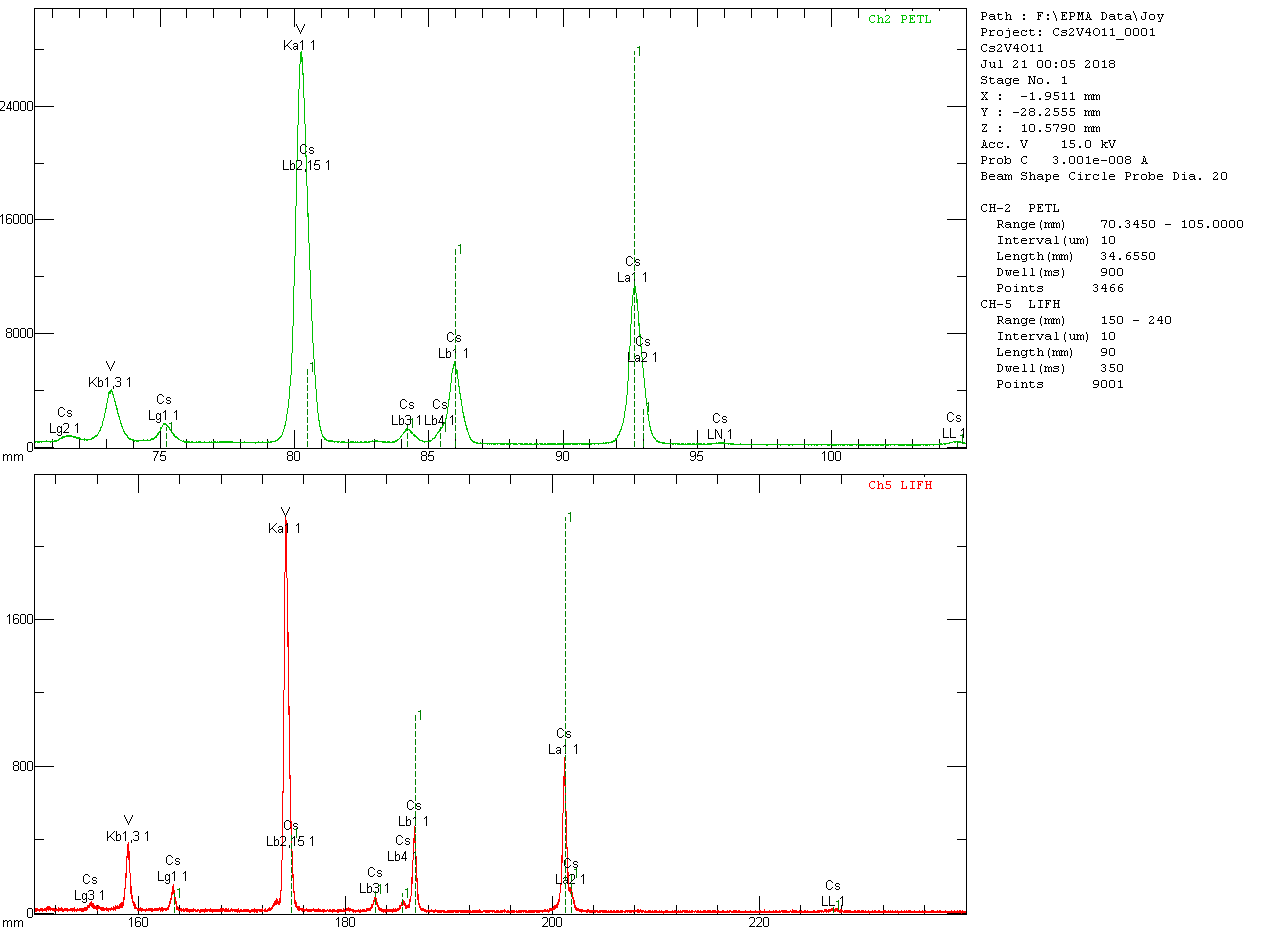
Note that Cs Lβ
2 interferes at the V Kα peak position using either PET or LiF.
Although I currently regard CsTiOAsO
4 as
the Cs standard (and I still want to synthesize CsNbOB
2O
5 and CsMnPO
4), Cs
2V
4O
11 is so easy to produce that it shouldn’t be disregarded.