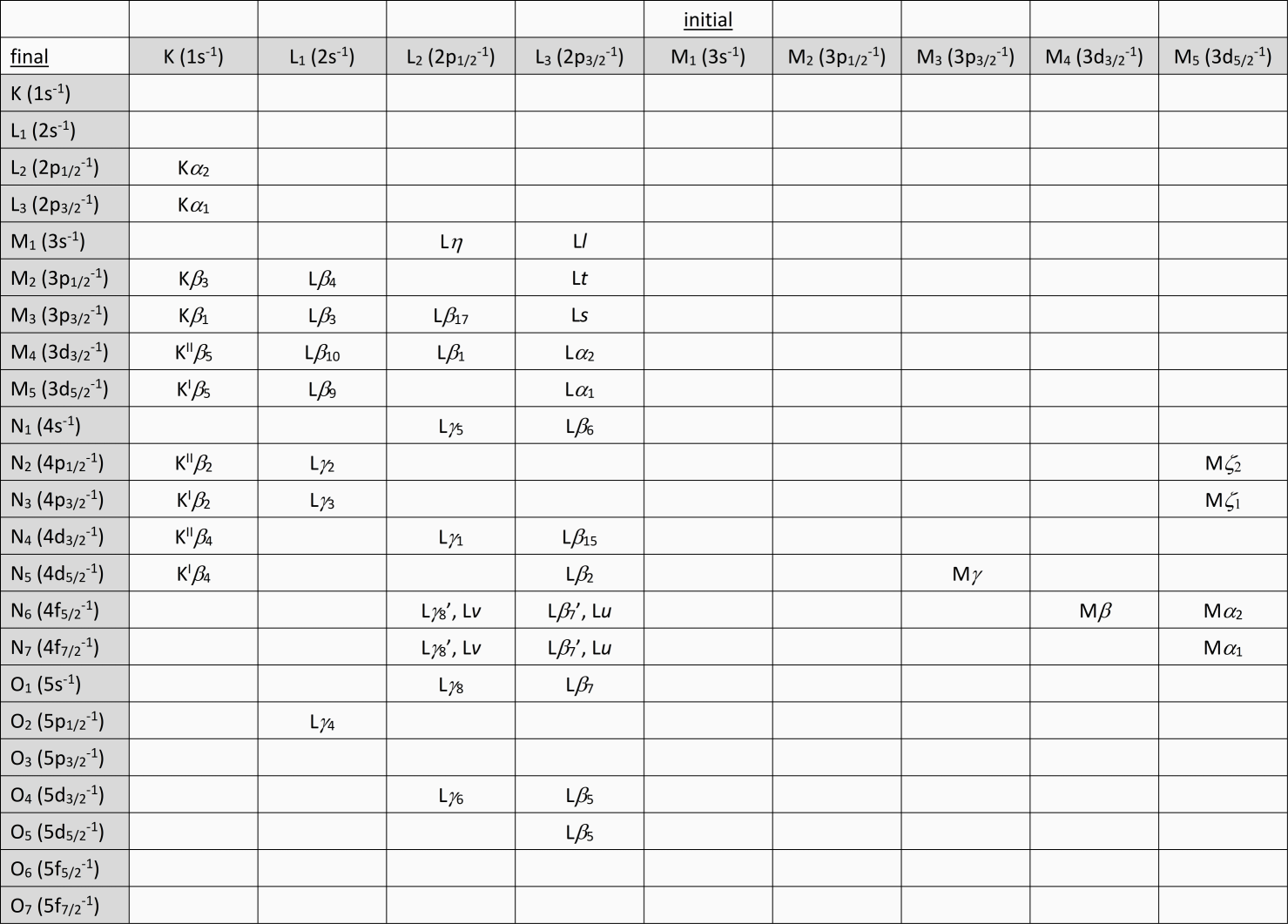
During the recent discussion of Ti K X-ray lines, I found myself getting confused as to which electronic transitions produced the Kβ
1, Kβ
2, Kβ
3, Kβ
4, and Kβ
5 X-ray lines. (Note that the ground state electronic configuration of Ti is [Ar]3d
24s
2.) So I’ve put together a table that shows the various transitions along with the Siegbahn notation for the X-ray line that represents the energy lost in the transition (e.g., Kβ
1,3 [Siegbahn] = K-M
2,3 [IUPAC]). The table below is attached as a Word file as well.
Could I get some feedback on this table? In particular, I’m a little confused on the transitions that produce the Mζ
1 and Mζ
2 X-rays. For instance, according to IUPAC, Mζ = M
4,5-N
2,3 with no distinction between Mζ
1 and Mζ
2 (though these lines are in fact EPMA resolvable).