I would just like to point out that although the Remote server is very useful for creating custom applications for your JEOL or Cameca instrument, for example see the sample Excel spreadsheets in the Remote.msi installer, e.g.,
TestRemote.xls - simple example of how to connect, and control your microprobe from Excel
TestReproduce.xls - example of calls to test the spectrometer reproducibility on your microprobe
TestStability.xls - example of calls to test your instrument stability over time
TestColumn.xls - example to control the column functions on your microprobe
The above Excel files will probably need to be changed to Excel "macro" files with the extension .xlsm because the Remote interface utilizes the macro feature in Excel. You can get started writing your own Excel macros to control your microprobe instrument by downloading the Remote server installer Remote.msi here:
https://probesoftware.com/smf/index.php?topic=88.msg315#msg315One can also develop custom image acquisition applications as described here:
https://probesoftware.com/smf/index.php?topic=754.0Finally I should note (the purpose of this post is), that although there is also a Deadtime_Acquire.xlsm Excel macro file that can be used to calibrate one's dead time constants, which utilizes the Deadtime_Calc.xlsx Excel spreadsheet designed by Paul Carpenter, I no longer recommend the use of these spreadsheets for the calibration of dead times on EPMA instruments.
The reason is that we now have a much more accurate dead time method which can handle the non-linear response of our counting electronics at much higher count rates as described here in this topic:
https://probesoftware.com/smf/index.php?topic=1466.msg11102#msg11102This new logarithmic dead time method published last year in Microscopy & Microanalysis allows one to exceed the linear response model that previously limited us to ~50 kcps and allows one to obtain stable count rates to ~300 to 400 kcps using the new constant k-ratio method as shown in our M&M paper
https://academic.oup.com/mam/article-abstract/29/3/1096/7165464If you do not have Probe for EPMA, the link above describes how to acquire the intensity data for this new dead time calibration, but if you do have Probe for EPMA, this new dead time calibration is very easy and fully automated, as described in the pdf file available from the Help menu in Probe for EPMA:
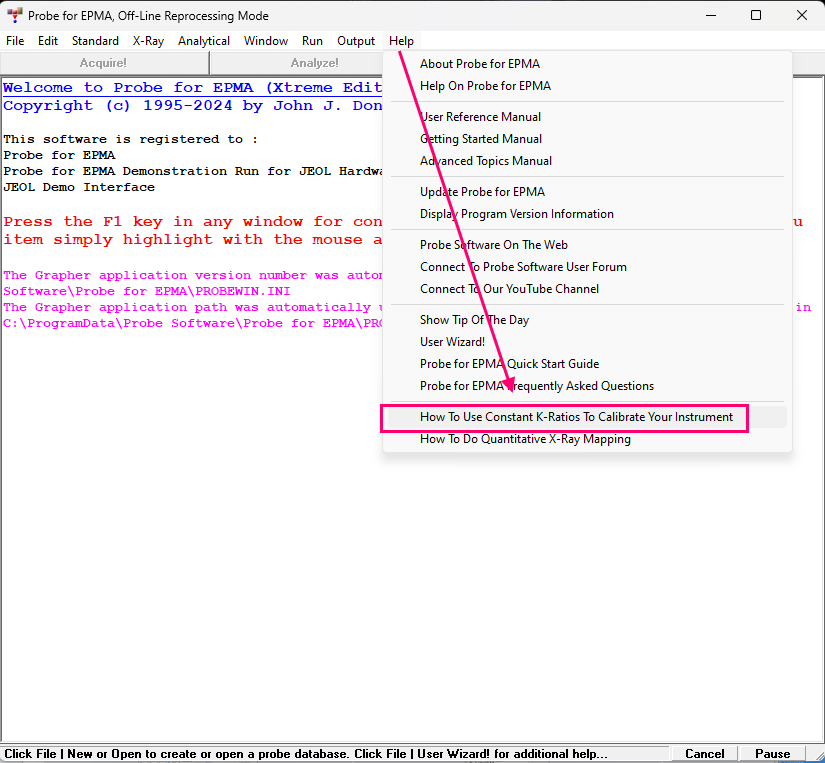


 If you are a member, please feel free to add your website URL to your forum profile
If you are a member, please feel free to add your website URL to your forum profile Recent Posts
Recent Posts