1
Probe for EPMA / Re: Correction of negative result in quant
« Last post by Probeman on Today at 09:07:43 AM »Here's a slide from my recent webinar on improving accuracy and sensitivity of trace element analyses:
https://www.youtube.com/watch?v=9KM5lU403VY&t=1478s

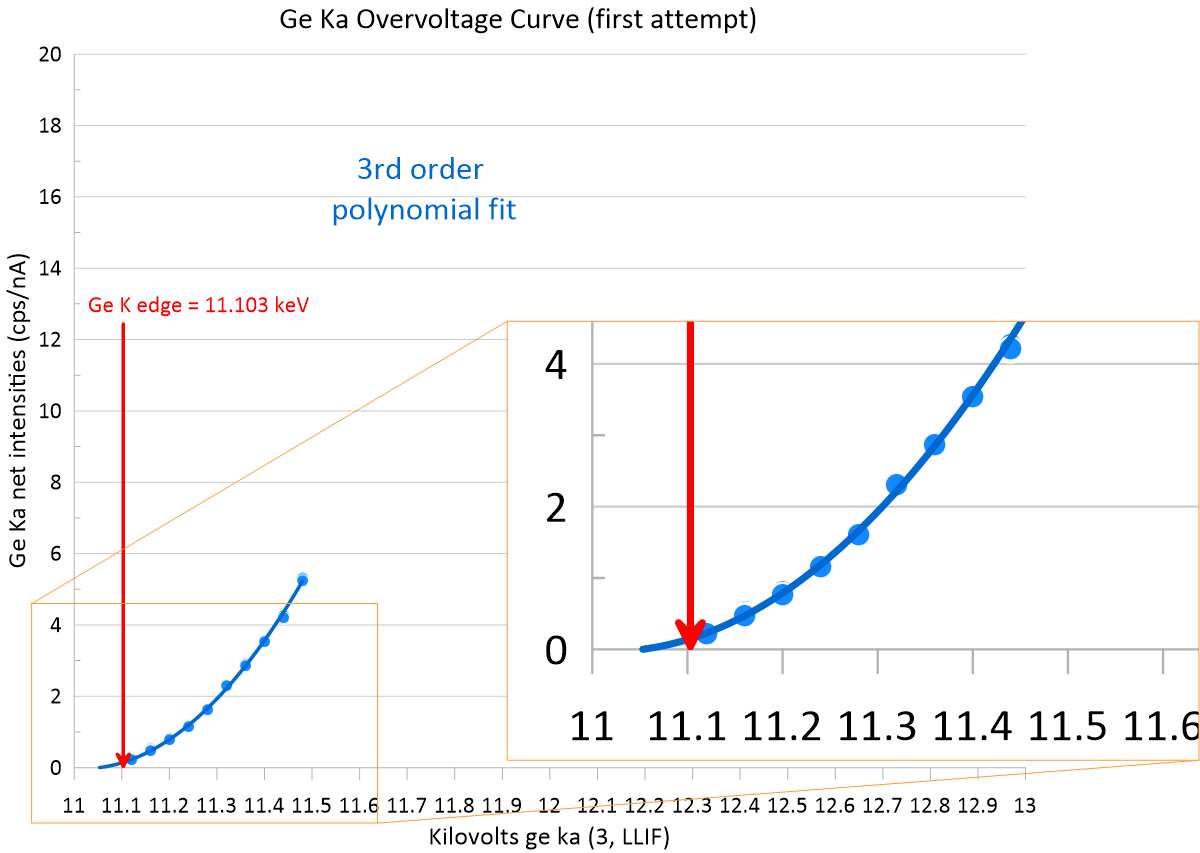
This slide is based on a slide sent to me by one of our commercial EPMA colleagues who asked to remain anonymous, but I think it makes the point well, that we should not be zeroing our negative intensities when measuring trace elements close to zero. If you are seeing consistently negative intensities, instead figure out what is wrong with your analytical approach. Maybe you have:
1. a spectral interference on one (or both) of your off-peak positions (check a wavescan and adjust your off-peak positions)
2. a curved background and you are using a linear interpolation (switch to a curved background model, e.g., exponential or polynomial)
3. perhaps you have a "hole" in your background under your on-peak position (apply a blank correction)
Regarding the last point, note that there are two types of "holes" in the continuum. One is due to an instrumental artifact, e.g., the hole under the Ti Ka peak position on some PET crystals, the other is due to an absorption edge in the sample, e.g., measuring Au Ma in iron sulfides. The latter requires a zero blank sample that (roughly) matches your unknown matrix, the former could be any high purity material since the artifact will show up in all materials (though in the case of Ti Ka, high purity SiO2 is readily available).
The discussion of this issue is found at time stamp: 47:05 in the webinar video.
https://www.youtube.com/watch?v=9KM5lU403VY&t=1478s

This slide is based on a slide sent to me by one of our commercial EPMA colleagues who asked to remain anonymous, but I think it makes the point well, that we should not be zeroing our negative intensities when measuring trace elements close to zero. If you are seeing consistently negative intensities, instead figure out what is wrong with your analytical approach. Maybe you have:
1. a spectral interference on one (or both) of your off-peak positions (check a wavescan and adjust your off-peak positions)
2. a curved background and you are using a linear interpolation (switch to a curved background model, e.g., exponential or polynomial)
3. perhaps you have a "hole" in your background under your on-peak position (apply a blank correction)
Regarding the last point, note that there are two types of "holes" in the continuum. One is due to an instrumental artifact, e.g., the hole under the Ti Ka peak position on some PET crystals, the other is due to an absorption edge in the sample, e.g., measuring Au Ma in iron sulfides. The latter requires a zero blank sample that (roughly) matches your unknown matrix, the former could be any high purity material since the artifact will show up in all materials (though in the case of Ti Ka, high purity SiO2 is readily available).
The discussion of this issue is found at time stamp: 47:05 in the webinar video.


 Welcome to the Probe Software forum area!
Welcome to the Probe Software forum area! Recent Posts
Recent Posts