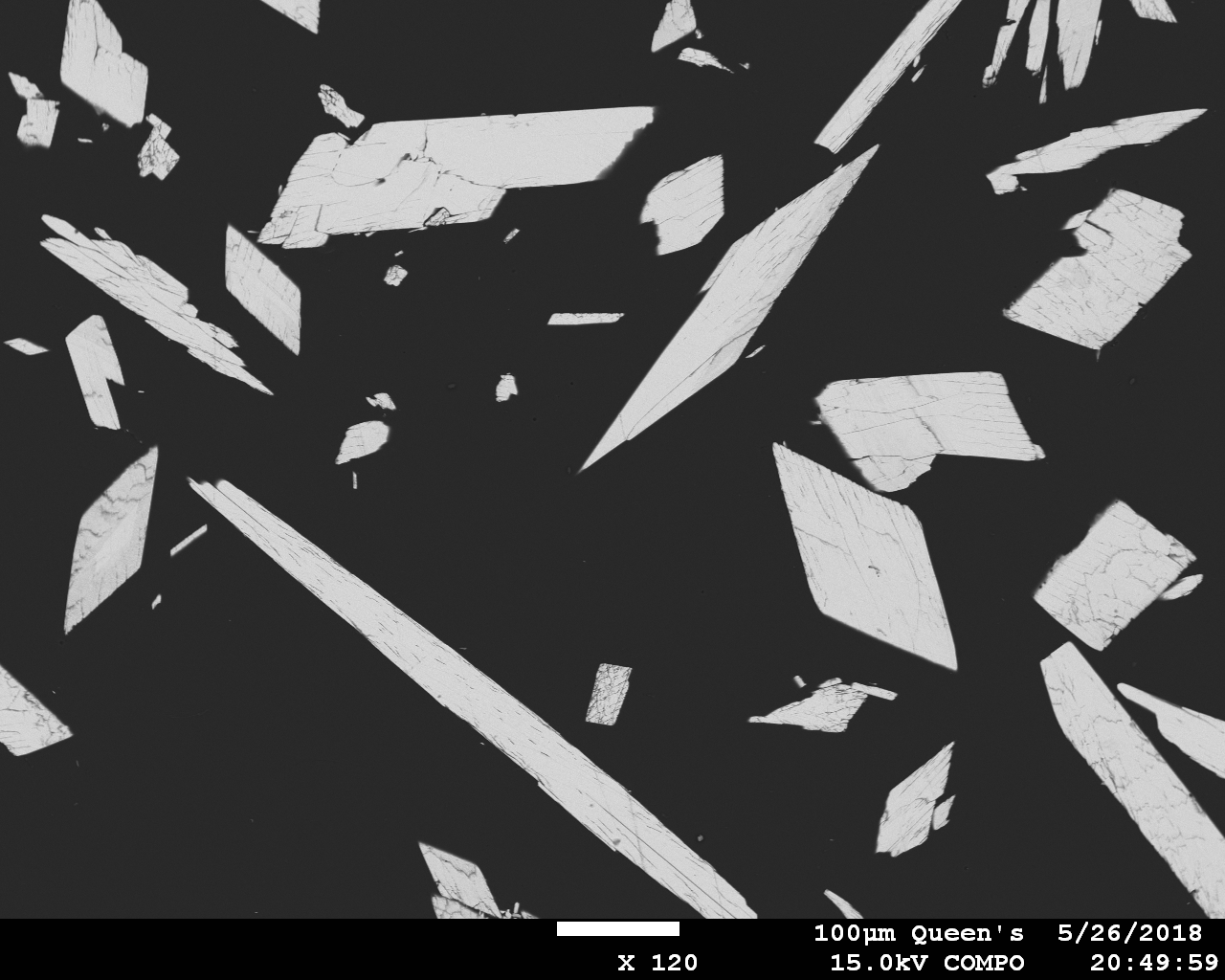
I’ve attached a couple of BSE images of the Sr-Cs phosphate that I produced in my first run.
The compound has symmetry no higher than monoclinic and appears to be the only phase present aside from a few grains of a hydrous Cs-Al phosphate (due to use of an alumina crucible). It possesses at least two perfect cleavages, and these cause some problems when polishing randomly oriented grains. Both simple and polysynthetic twins appear to be present. The compound has been boiled, ultrasonicated, ground, and polished in H2O and shows no evidence of solubility in it.
I’ve analyzed it quantitatively, but the combination of a lack of a reliable Cs standard and significant atomic number and/or absorption corrections for Sr and P make me reluctant to assign a formula unit at this point. What I’m willing to state is that the formula unit is likely bounded by Sr
2Cs
3(PO
3)
7 and Sr
3Cs
5(PO
3)
11. The first formula corresponds to wt% SrO = 18.39, wt% Cs
2O = 37.52, and wt% P
2O
5 = 44.09, while the second, wt% SrO = 17.31, wt% Cs
2O = 39.23, and wt% P
2O
5 = 43.47.
The compound is beam sensitive and shows clear evidence of Cs migration under a focused beam, even at 2-3 nA beam current. When I performed the analyses, I used a 15 kV potential, 5 nA current, defocused the beam to 10 microns, and counted for 10 s peak and a total of 10 s background. I intend to analyze it again at different beam currents to see if the results differ significantly, but I won’t be able to do this until late in the week.
The initial thought was that the topology of the phase diagram for the Sr(PO
3)
2-CsPO
3 system should mimic that of the Ba(PO
3)
2-CsPO
3 system. In the latter system, the two intermediate compounds are BaCs
4(PO
3)
6 and Ba
2Cs(PO
3)
5. So either I’ve produced something metastable, or the topology of the Sr(PO
3)
2-CsPO
3 system departs from that of the Ba-bearing system. I have a second run in the furnace right now. I kept the first one in the furnace for 22 hours, and I’ll let this one run for perhaps ~100 hours just to see if the results are time-dependent. If I get the same results, I’ll try to grow larger crystals in run 3. I’ll likely try a run in the Ba(PO
3)
2-CsPO
3 system for comparison.