Hi John and all
Not sure if it’s of any interest, but just thought I would post some of my failures in the last week trying to measure C in scapolite on our SXFive without any form of anticontamination…
Firstly after doing some separate measurements for F we decided that there was little to no F in the scapolite we were looking at, so could ditch F from the package and run C with the rest of the elements to get around the combined analyses problem
In regards to the coating, I tried both 5 and 10nm of Ag coat. I get ~1.5 times the counts with a 5nm coat on my scapolite NMNH standard (see wavescans below – ignore the fact that my PC2 xtal is wayyyy out of alignment, that major peak is Oxygen, the carbon peak is the small broader hump to the left of it).

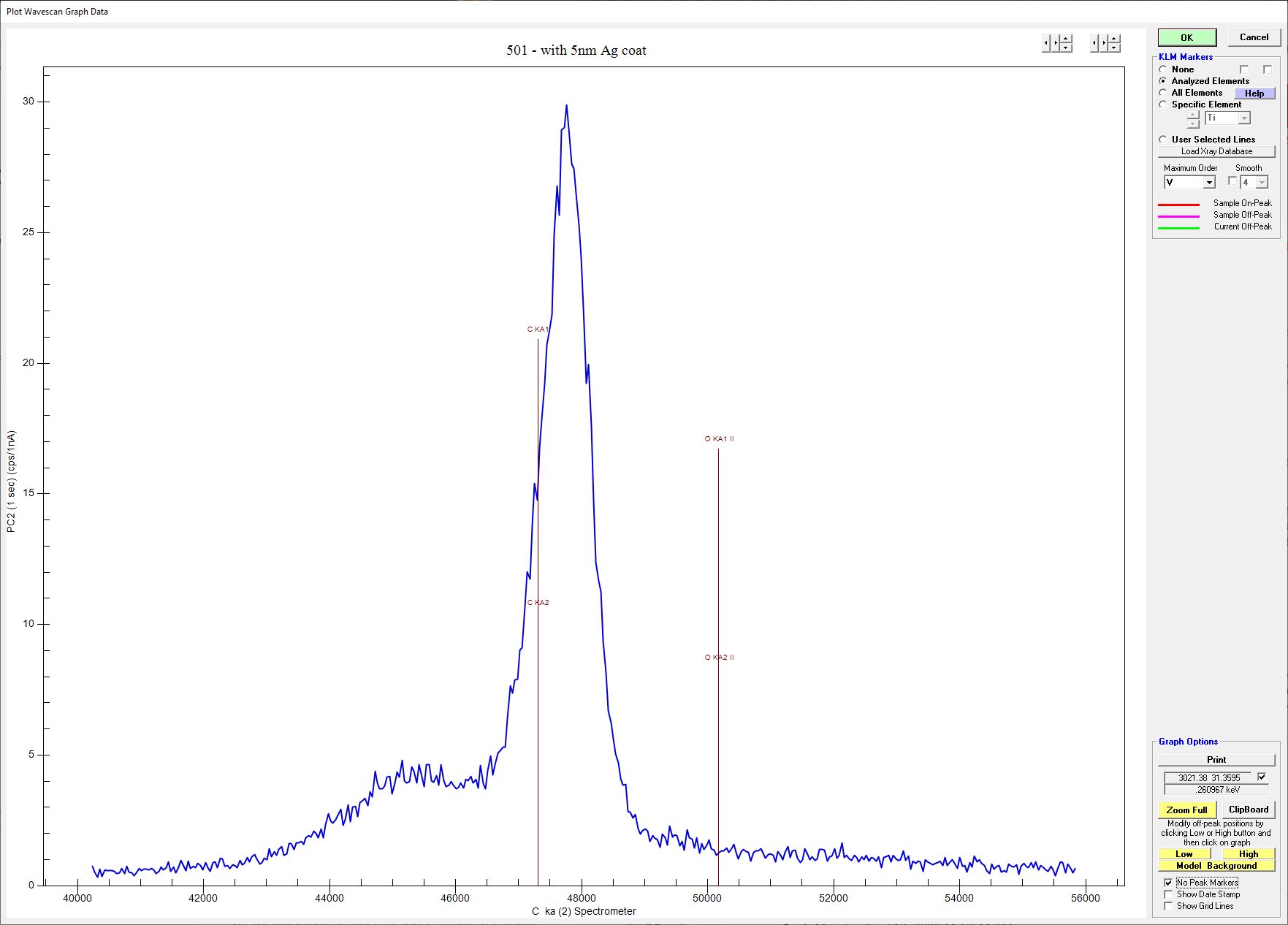
The 5nm seemed to work fine with regards to negating charging so I ended up going with that. To get around having to use coating corrections between standards and unknowns, I ended up finding an older Astimex mineral mount laying around so polished the carbon coat off and coated it in Ag at the same time as the unknowns and NMNH scapolite mount. Despite me thinking I polished the life out of the samples, getting the samples to the coater quickly and keeping it all under vacuum, I had real problems with C still being measured in minerals that should have none. Wavescan below shows C peak in standard 501 albite.
Some of my measured C in standards are probably interferences, as seen in MAN fit diagram below for C. I am sure some of it is residual carbon coat/adsorbed carbon to the sample surface, which I guess isn’t unexpected. If I concentrate on the lower Z end where scapolite will plot (mean Z ~11.6), I can refine the fit to something OK but am not left with many data points (refined MAN fit plot below). I am hoping that these standards left perhaps don’t have as much residual carbon on their surface and this correlation is real and mostly due to mean Z. Using the MAN vs traditional, I get ~10% higher carbon values on unknowns so perhaps there is still some other extraneous C contribution.


Despite this I ended up running a standards and unknowns at 10kV/35nA/10um defocussed beam. Both standards and unknowns had TDI enabled. Standards were collected with traditional background methods, and unknowns both with and without MAN for comparison. TDI plots on unknowns were variable, some spots showing C increase which might be expected, some showing no correlation at all (attached images).


I ended up using NMNH R6600 Scapolite as my Carbon standard for the unknowns. I tried pure graphite, calcite, dolomite, and all give me extremely high C measurements when treating NMNH R6600 Scapolite as an “unknown” (reported CO2 is 2.5 wt%, returned values range from 7-10wt% when using dolomite/calcite/graphite).
Given the inherent “background” level of carbon, I decided to utilise the blank assignment. For the levels of C I am measuring in my unknowns (<3 wt% C), applying this correction cuts my C concentrations in half, from ~2.5wt% without correction to ~1.2 wt% with. This is due to the quite large “background” contribution and the low amount of counts we are dealing with.
Unfortunately in the unknowns there is no other mineral of similar major element composition to the scapolite, there being only hornblende +/-kspar and biotite in the rock. They have similar C background levels, but are subtlety different assuming related to their mean-Z [K-spar (z avg 11.67/3.69 CPS/nA); hornblende (z avg 13.15/ 4.34 CPS/nA)]. Given scapolite has a mean-Z of ~11.65, the best I could do is use k-spar if I could find it, otherwise I used hornblende. Depending on which mineral you use, there is a ~0.13 wt% difference in the analysed unknowns, so not trivial.
So one question I have is this….I assume my C standard (NMNH R6600 Scapolite) also has this “background” level of carbon on top of its structural carbon? Should I be doing some kind of blank correction for this as well? We aren’t talking about a lot of counts to work with here either. An average of 75 analyses of the NMNH R6600 Scapolite returns 7.66 +/- 0.50 CPS/nA, and this is on something that has 2.5 wt% CO2. So not a lot to work with in the first place, and when you look at the “background” levels of C on various other “C-free” standards like albite/plag/sanidine it ranges from 3.0-4.5 CPS/nA. So not really sure how to treat my C standard with regards to blank subtraction at this stage.
So I am at the point where I can get some analyses on scapolite with OK totals and varying C from sample to sample (see Results image of combined samples KM31 and KM22 below), but have no real confidence if those C contents are real or not. What I need is more scapolite standards I think to do more cross checking, it’s all a bit circular at the moment.
That is my ramble fest over. I will keep you updated and any suggestions are gratefully received.

Cheers