From time to time, I rant about units; I do this because they’re an essential part of the quantity being considered. In addition, failure to keep track of units during a calculation removes an important check on the validity of the result of the calculation. Below I’ll work through the units of the Armstrong
ϕ(
ρz) model, which is derived from that of Packwood and Brown (1981, X-ray Spectrometry 10: 138-146, attached).
Let me start by writing out the Armstrong
ϕ(
ρz) model as contained in
Electron Probe Quantitation ("the Green Book"), p. 269-271. I’ve also attached the original reference from 1982.
For a given X-ray (no subscript) in a mixture consisting of elements
i, where
C,
Z, and
A are respective concentration, atomic number, and atomic weight, where
E0 and
Ec are, respectively, beam energy and critical excitation energy in keV, and where
U0 =
Ec/
E0,

Mean ionization energy of Berger and Seltzer (1964, NASA SP-3012), energy in keV:

Surface ionization function of Love
et al. (1978, J. Phys. D, 11: 23-31):
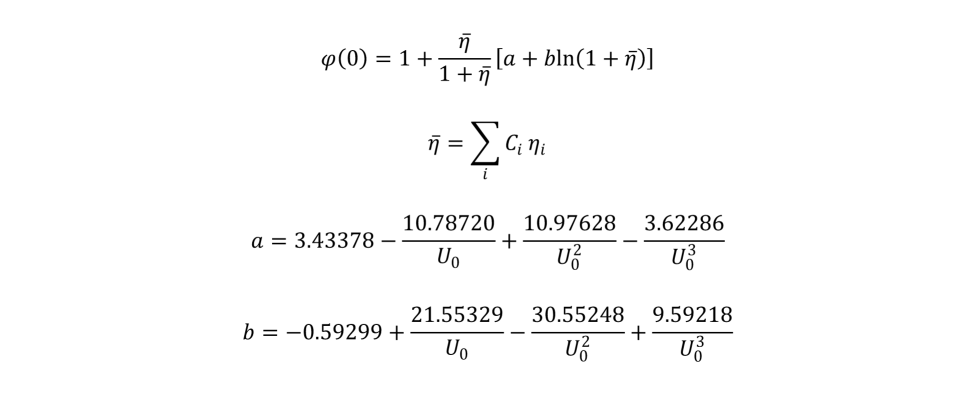
Electron backscatter coefficient of Love and Scott (1978, J. Phys. D, 11: 1369-1376), energy in keV:

Starting with the equation for
ηi, note that the “20” appears to carry no units; referring to Love and Scott (1978, J. Phys. D 11:1369-1376), the “20” should actually be “20 keV.” Taking this into account, the resulting value for
ηi is dimensionless, as it should be. Since
U0 is a ratio of energies,
ϕ(0) and
γ0 are also dimensionless.
In the expression for
β, all quantities are dimensionless except for the beam energy,
E0 [keV] and the mean atomic mass,
A [g mol
-1] (implying that
ρ should be in units of [g cm
-3]). The units for
β are [mol g
-1 keV
-2]. In the expression for
ϕ,
β is multiplied by
ρz, which carries units of [g cm
-2], and so -
βρz, the argument of an exponential function, has units of [mol cm
-2 keV
-2]. That’s a problem 1) because the term containing the exponential function is subtracted from unity and 2) because exponentiation wreaks havoc on the units.
The exponential function containing
α in its argument also must yield a dimensionless result, noting that
ϕ(
ρz) itself is dimensionless. One can see easily that this condition cannot be met due simply to the fact that, in the expression for
α,
E0 is raised to a fractional exponent, 1.25 (Packwood and Brown, 1981, eq. 14). The resulting units for
α are [mol keV
-1.75 g
-1]. Note that, in allowing this exponent to vary (from unity), Packwood and Brown also allowed the units to vary. One must be careful to avoid such a situation when working with fractional exponents.
A different way to look at things would be to assume that the constants in the expressions for
α and
β carry units such that the result of each calculation is dimensioned correctly. If this is the case, and excluding any variation in units, then the apparently dimensionless 2.97·10
5 in the expression for α is actually 2.97·10
5 keV
1.75 g mol
-1, while the constant in the expression for
β would need to be written as 8.5·10
5 [g KeV
2 mol
-1] * [cm
2 g
-1] = 8.5·10
5 cm
2 keV
2 mol
-1.
One might argue that only the numerical result is important. But that’s just not true; the number and the unit(s) are bound to each other, at least until normalized/non-dimensionalized properly, and, even then, a link still exists. The units have to make sense, and, if they don’t, the numerical result can’t either.